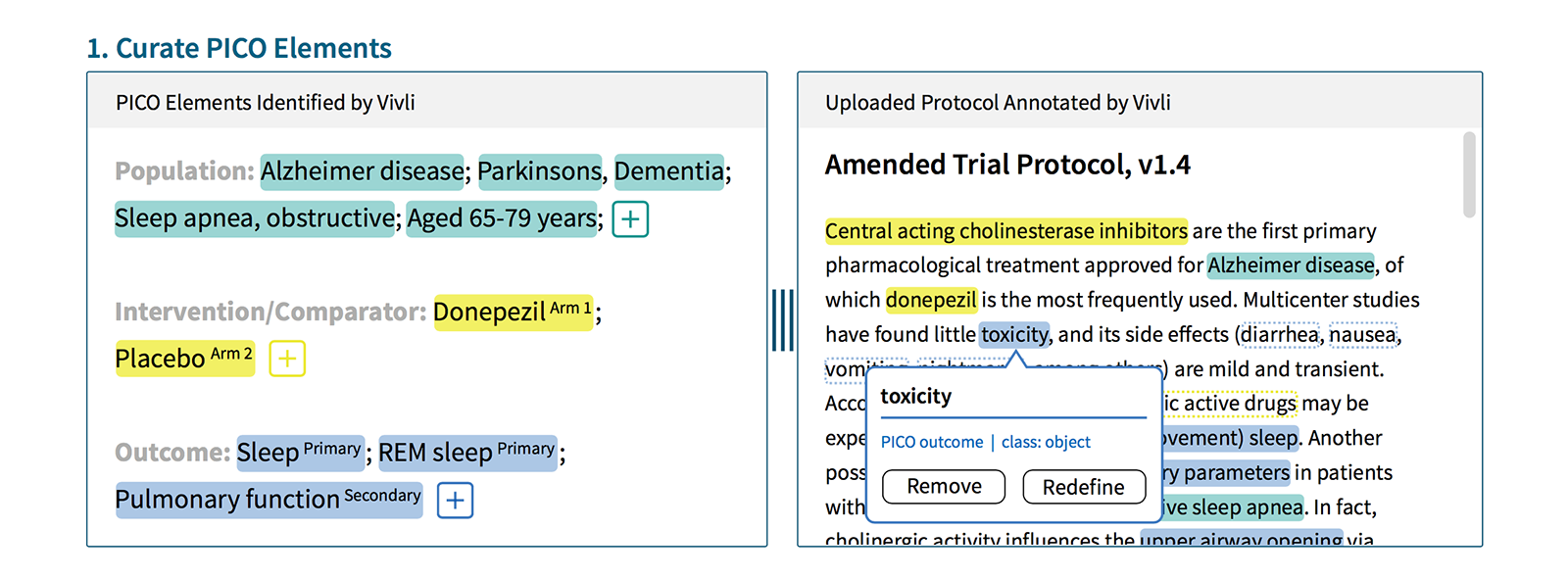
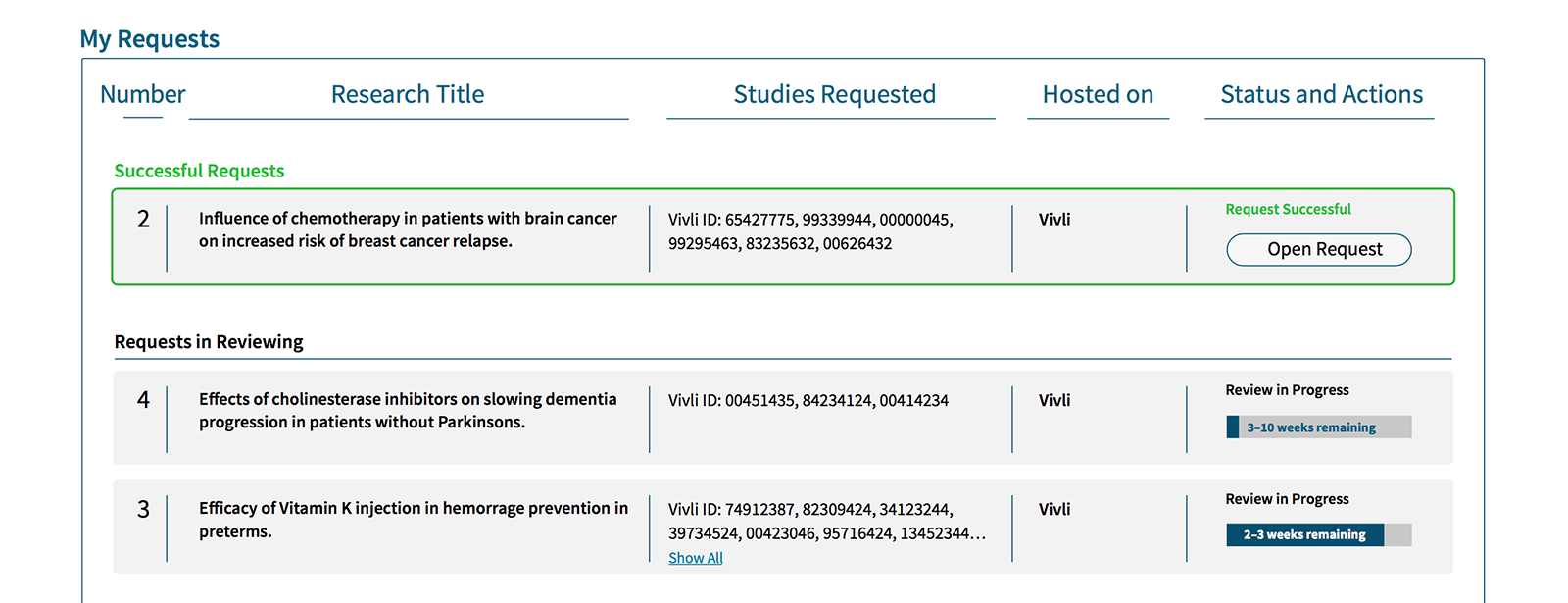
Our aim was to design, prototype, and test an end-to-end Vivli experience for two types of user:
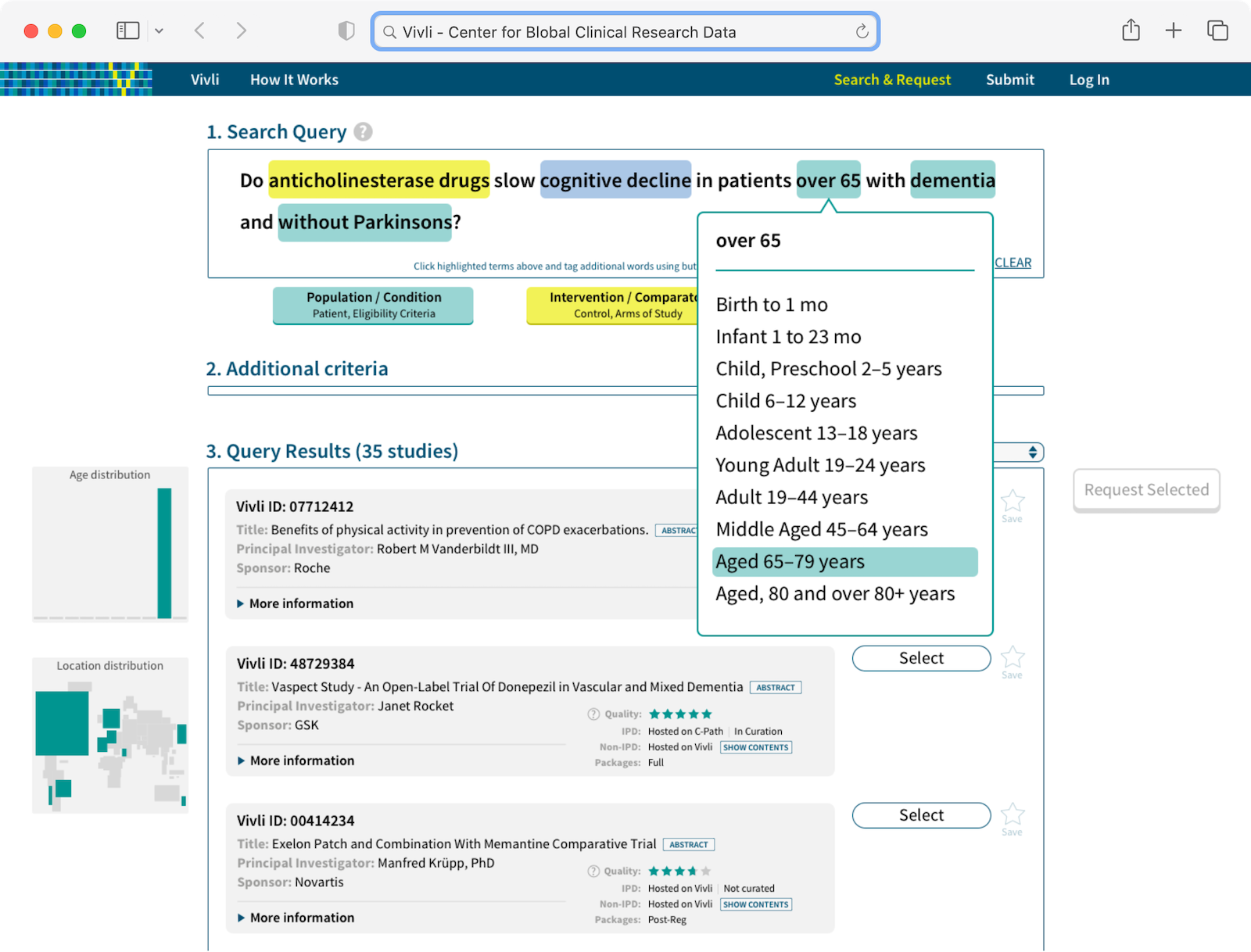
- Requesters, who would use Vivli to search for studies and request access to individual patient data
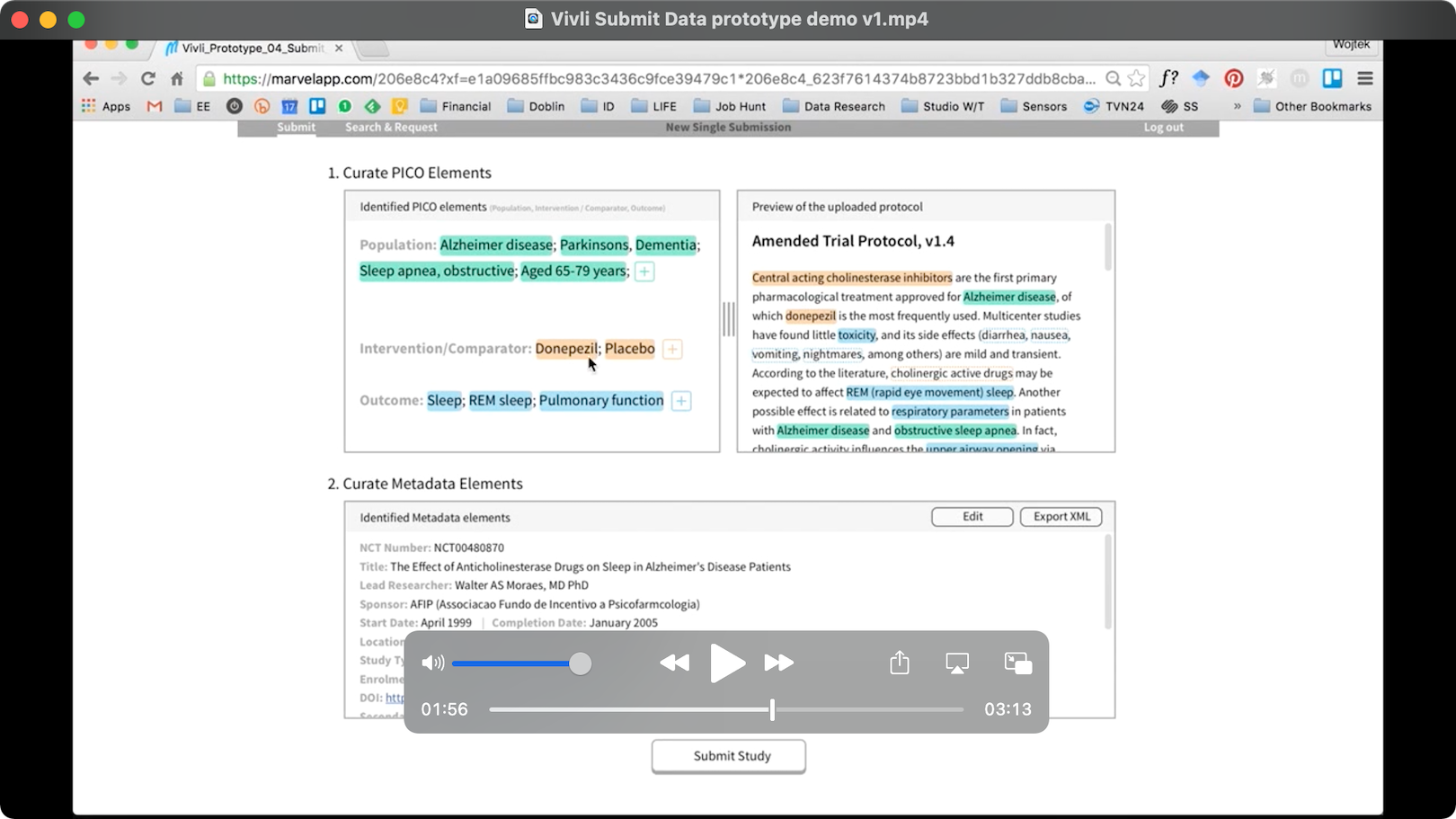
- Submitters, who would use Vivli to add studies to the Vivli catalogue for requesters to discover and use
We used prototypes as stimulus material in expert research that we conducted with thought leaders in information sharing platforms in healthcare. We also used our prototypes with Vivli's potential users working all over the world in various medical domains, such as: randomised clinical trials, evidence synthesis, clinical informatics, and secondary analysis.
Our findings helped us improve the prototype and refine the Vivli experience. The prototypes were also used as a tool for strengthening the Vivli consortium and communicating Vivli's vision to stakeholders and collaborators.
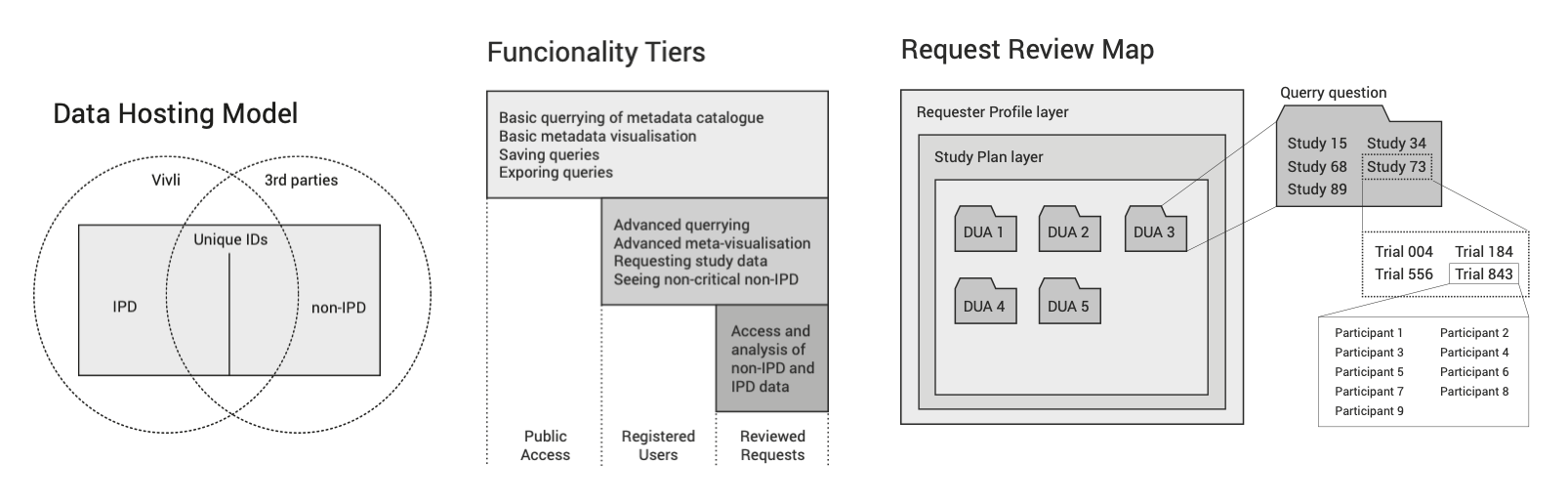
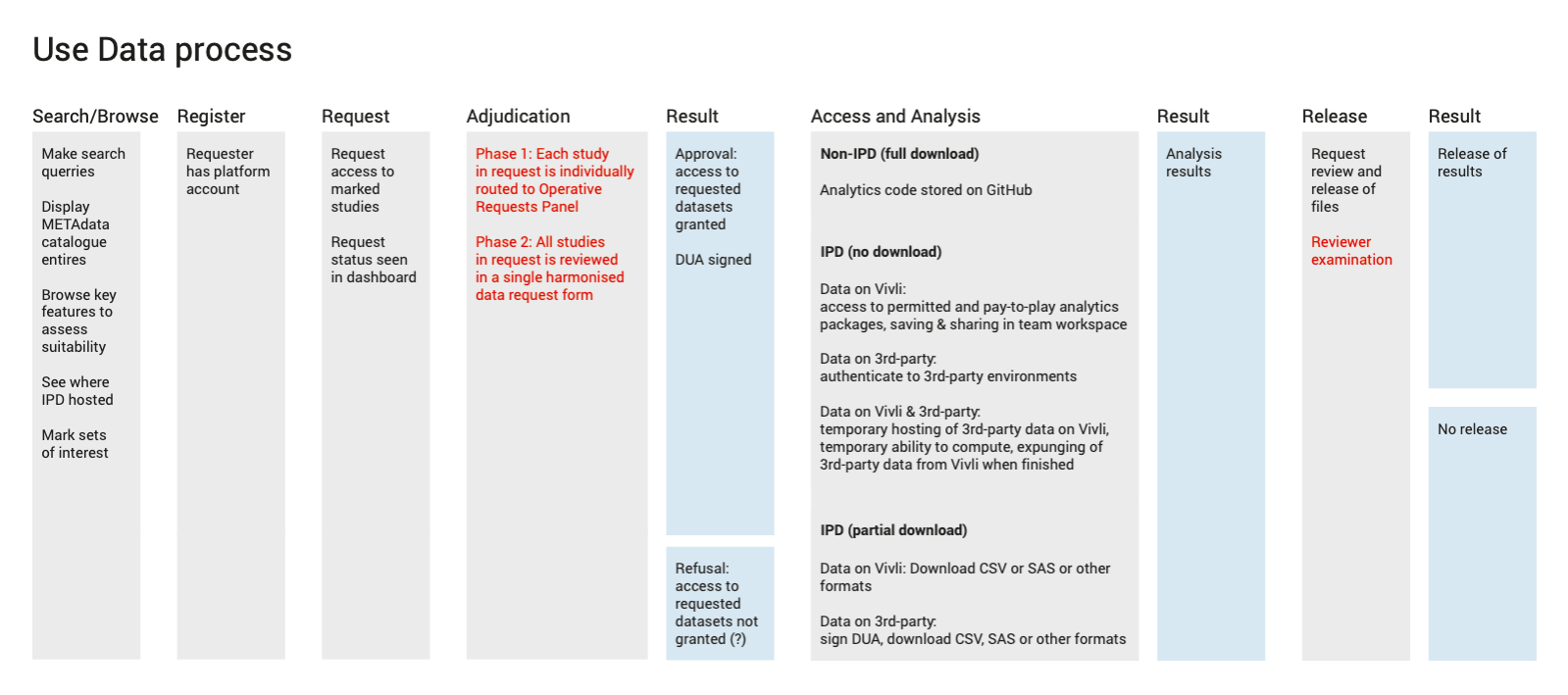
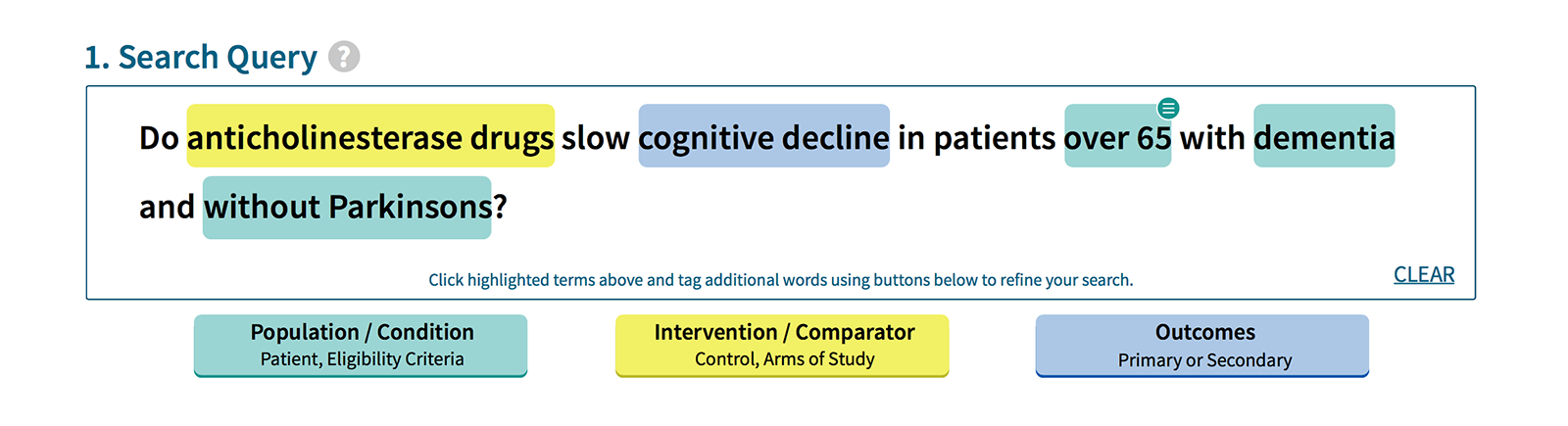
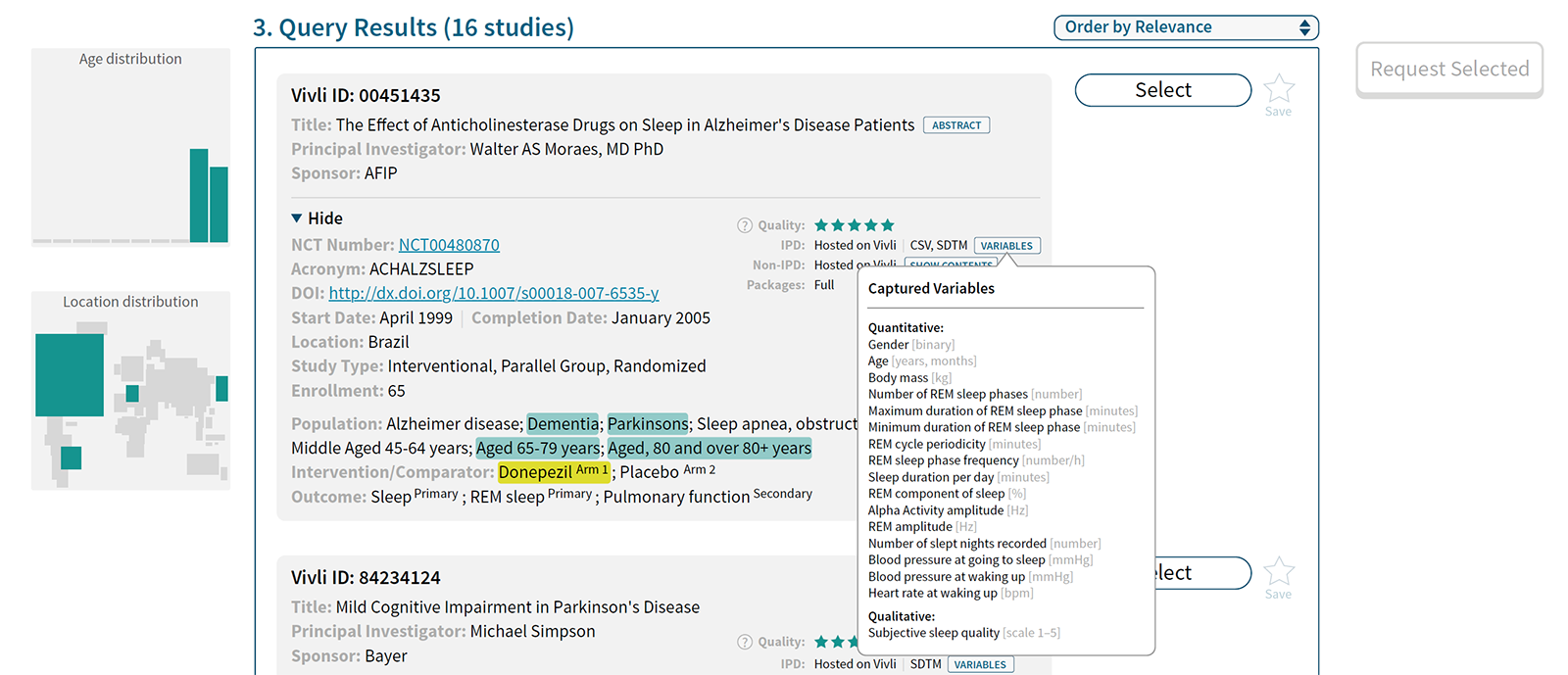
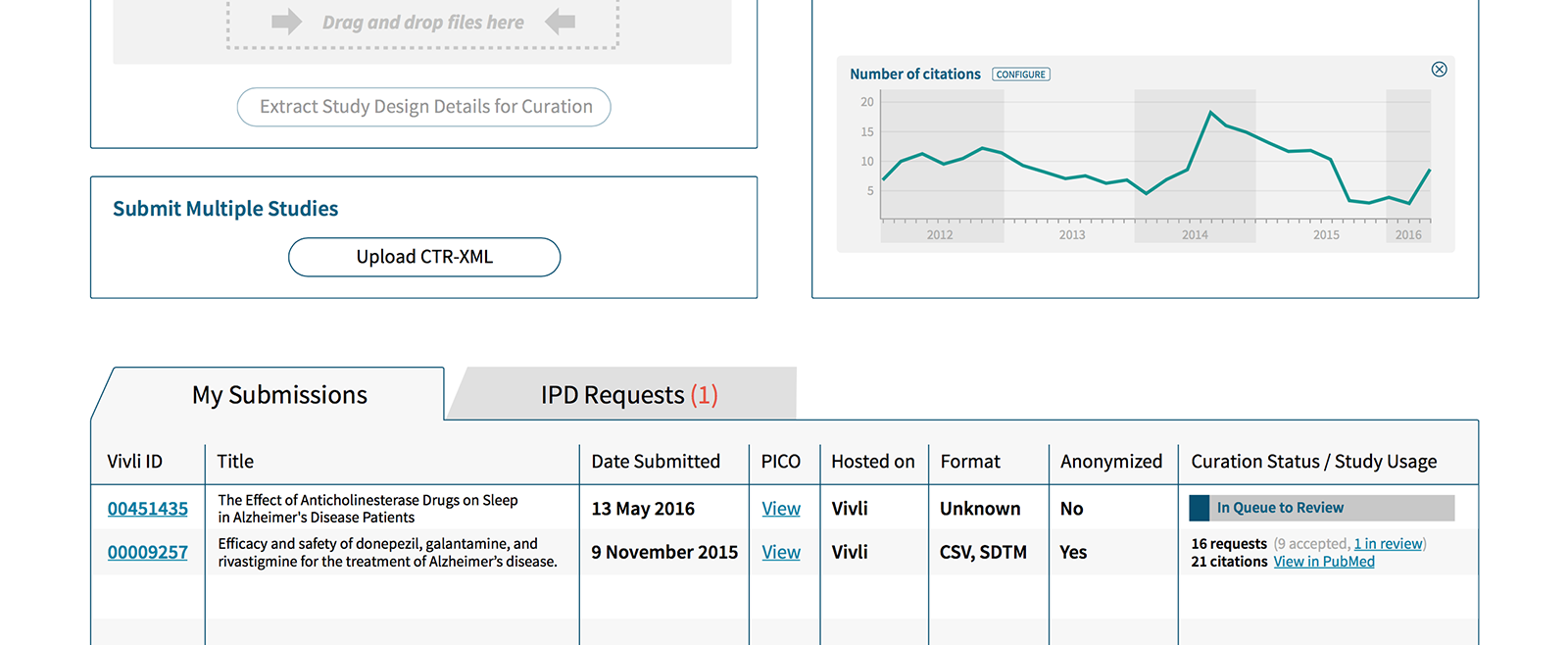
During our research we identified the challenges a data platform such as Vivli is going to face. Below are just a few of our findings that informed design decisions.